Kevin G. Cooper
Long acting progestogens versus combined oral contraceptive pill for preventing recurrence of endometriosis related pain: the PRE-EMPT pragmatic, parallel group, open label, randomised controlled trial
Cooper, Kevin G.; Bhattacharya, Siladitya; Daniels, Jane P.; Horne, Andrew W.; Clark, T. Justin; Saridogan, Ertan; Cheed, Versha; Pirie, Danielle; Melyda, Melyda; Monahan, Mark; Roberts, Tracy E.; Cox, Emma; Stubbs, Clive; Middleton, Lee J.; on behalf of the PRE-EMPT Collaborative Group
Authors
Siladitya Bhattacharya
Professor JANE DANIELS JANE.DANIELS@NOTTINGHAM.AC.UK
PROFESSOR OF CLINICAL TRIALS
Andrew W. Horne
T. Justin Clark
Ertan Saridogan
Versha Cheed
Danielle Pirie
Melyda Melyda
Mark Monahan
Tracy E. Roberts
Emma Cox
Clive Stubbs
Lee J. Middleton
on behalf of the PRE-EMPT Collaborative Group
Abstract
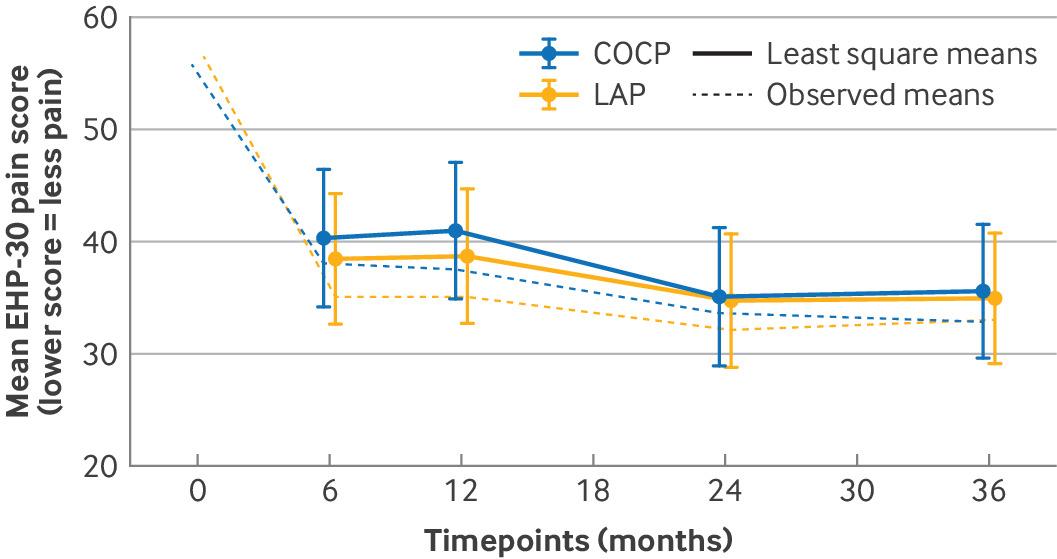
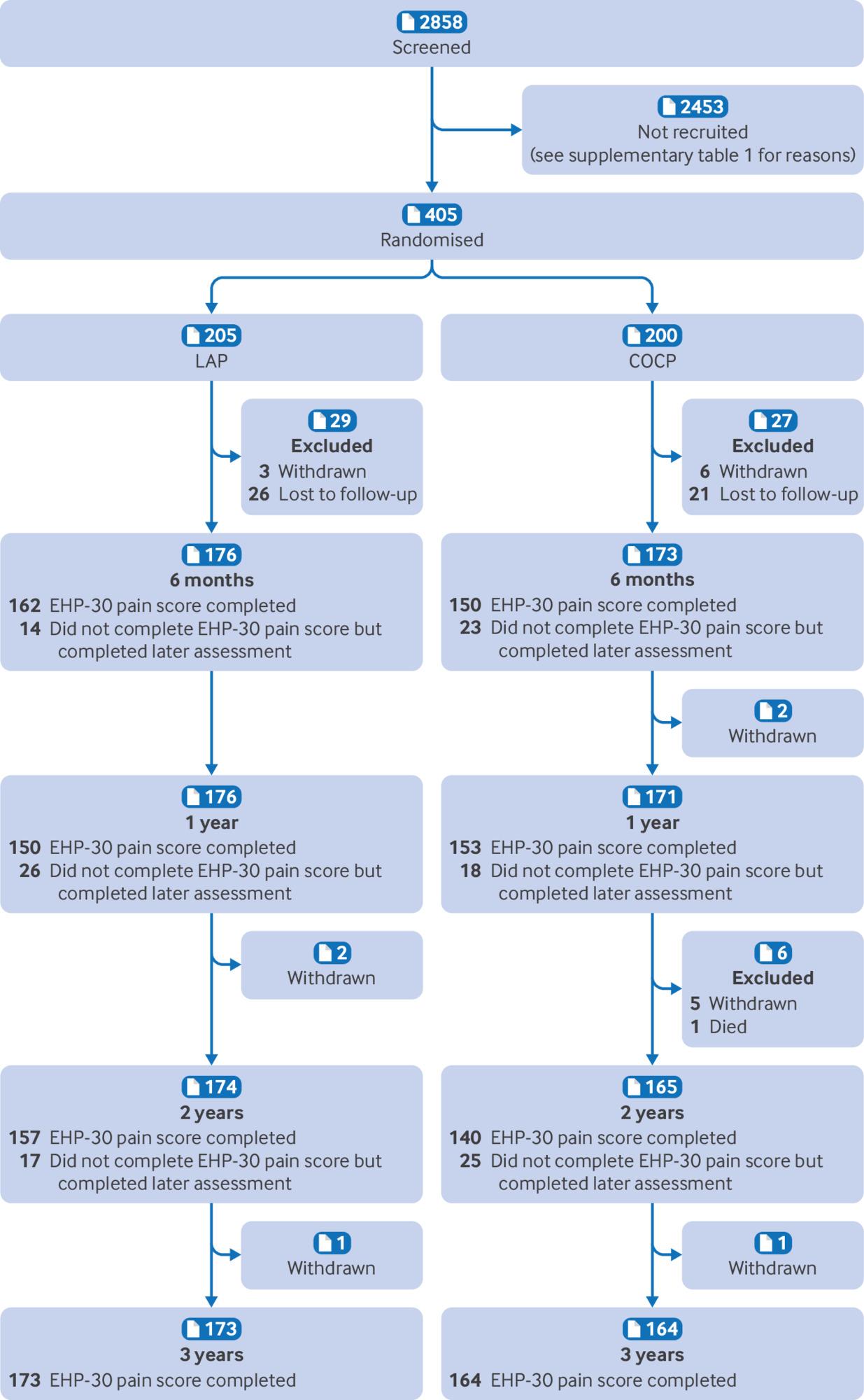
Objectives: To evaluate the clinical effectiveness of long acting progestogens compared with the combined oral contraceptive pill in preventing recurrence of endometriosis related pain. Design: The PRE-EMPT (preventing recurrence of endometriosis) pragmatic, parallel group, open label, randomised controlled trial. Setting: 34 UK hospitals. Participants: 405 women of reproductive age undergoing conservative surgery for endometriosis. Interventions: Participants were randomised in a 1:1 ratio using a secure internet facility to a long acting progestogen (depot medroxyprogesterone acetate or levonorgestrel releasing intrauterine system) or the combined oral contraceptive pill. Main outcome measures: The primary outcome was pain measured three years after randomisation using the pain domain of the Endometriosis Health Profile 30 (EHP-30) questionnaire. Secondary outcomes (evaluated at six months, one, two, and three years) included the four core and six modular domains of the EHP-30, and treatment failure (further therapeutic surgery or second line medical treatment). Results: 405 women were randomised to receive a long acting progestogen (n=205) or combined oral contraceptive pill (n=200). At three years, there was no difference in pain scores between the groups (adjusted mean difference −0.8, 95% confidence interval −5.7 to 4.2, P=0.76), which had improved by around 40% in both groups compared with preoperative values (an average of 24 and 23 points for long acting progestogen and combined oral contraceptive pill groups, respectively). Most of the other domains of the EHP-30 also showed improvement at all time points compared with preoperative scores, without evidence of any differences between groups. Women randomised to a long acting progestogen underwent fewer surgical procedures or second line treatments compared with those randomised to the combined oral contraceptive pill group (73 v 97; hazard ratio 0.67, 95% confidence interval 0.44 to 1.00). Conclusions: Postoperative prescription of a long acting progestogen or the combined oral contraceptive pill results in similar levels of improvement in endometriosis related pain at three years, with both groups showing around a 40% improvement compared with preoperative levels. While women can be reassured that both options are effective, the reduced risk of repeat surgery for endometriosis and hysterectomy might make long acting reversible progestogens preferable for some. Trial registration: ISRCTN registry ISRCTN97865475.
Citation
Cooper, K. G., Bhattacharya, S., Daniels, J. P., Horne, A. W., Clark, T. J., Saridogan, E., Cheed, V., Pirie, D., Melyda, M., Monahan, M., Roberts, T. E., Cox, E., Stubbs, C., Middleton, L. J., & on behalf of the PRE-EMPT Collaborative Group. (in press). Long acting progestogens versus combined oral contraceptive pill for preventing recurrence of endometriosis related pain: the PRE-EMPT pragmatic, parallel group, open label, randomised controlled trial. BMJ, 385, e079006. https://doi.org/10.1136/bmj-2023-079006
| Journal Article Type | Article |
|---|---|
| Acceptance Date | Apr 9, 2024 |
| Online Publication Date | May 15, 2024 |
| Deposit Date | Jun 25, 2024 |
| Publicly Available Date | Jul 2, 2024 |
| Journal | BMJ |
| Print ISSN | 0959-8138 |
| Electronic ISSN | 1756-1833 |
| Publisher | BMJ Publishing Group |
| Peer Reviewed | Peer Reviewed |
| Volume | 385 |
| Pages | e079006 |
| DOI | https://doi.org/10.1136/bmj-2023-079006 |
| Public URL | https://nottingham-repository.worktribe.com/output/35142002 |
Files
Long acting progestogens versus combined oral contraceptive pill
(552 Kb)
PDF
Publisher Licence URL
https://creativecommons.org/licenses/by/4.0/
Long acting progestogens versus combined oral contraceptive pill
(176 Kb)
Image
Long acting progestogens versus combined oral contraceptive pill
(177 Kb)
Image
Long acting progestogens versus combined oral contraceptive pill
(308 Kb)
Image
You might also like
Metformin for endometrial hyperplasia
(2024)
Journal Article
Downloadable Citations
About Repository@Nottingham
Administrator e-mail: discovery-access-systems@nottingham.ac.uk
This application uses the following open-source libraries:
SheetJS Community Edition
Apache License Version 2.0 (http://www.apache.org/licenses/)
PDF.js
Apache License Version 2.0 (http://www.apache.org/licenses/)
Font Awesome
SIL OFL 1.1 (http://scripts.sil.org/OFL)
MIT License (http://opensource.org/licenses/mit-license.html)
CC BY 3.0 ( http://creativecommons.org/licenses/by/3.0/)
Powered by Worktribe © 2025
Advanced Search